Support your cardiovascular patients
Complex cardiac surgeries involving CPB can be associated with a dysregulated immune response, which may contribute to complications such as vasoplegic shock and multi-organ dysfunction. Emergency cardiac surgeries in patients receiving antithrombotic drugs such as Ticagrelor or Rivaroxaban carry an increased risk of perioperative bleeding. CytoSorb® is approved for removal of Cytokine and during cardiopulmonary bypass, Ticagrelor and Rivaroxaban.
Support hemostatic control in urgent surgeries
Intraoperative use of CytoSorb® is intended for the removal of ticagrelor and rivaroxaban during cardiopulmonary bypass, aiming to support hemostatic management during urgent surgical interventions.
Managing bleeding risk in urgent cardiac surgery
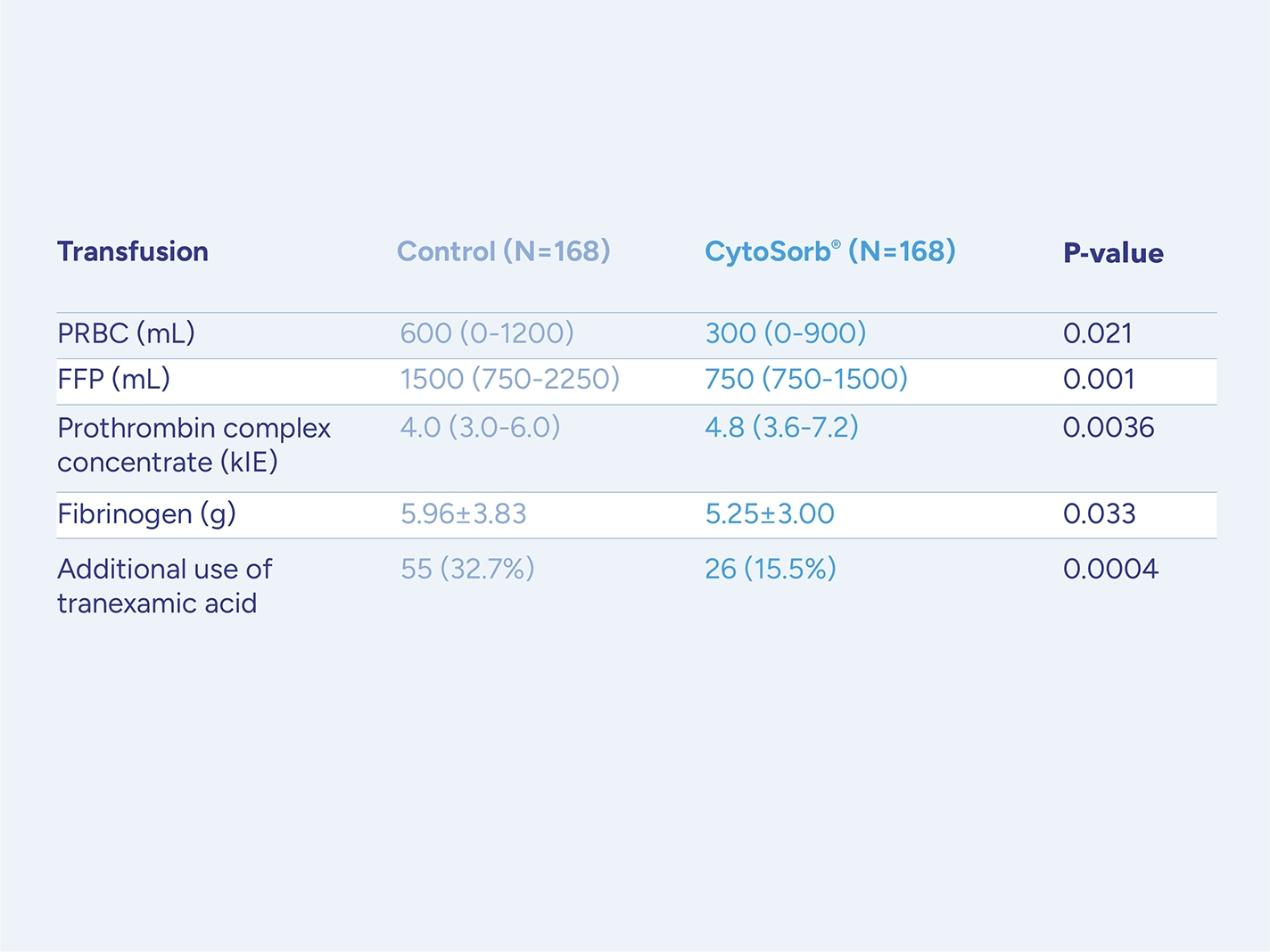
Patients on antithrombotic drugs who require urgent or emergent cardiac surgery and cannot wait for drug washout face a high risk for serious perioperative bleeding complications.
CytoSorb is approved for use during CPB in patients on ticagrelor or rivaroxaban undergoing urgent or emergent cardiac surgery with the goal of reducing the severity of perioperative bleeding.

Best practice therapy management
Support surgical care and inflammatory control
Major surgery with CPB can be associated with postoperative inflammatory responses that may contribute to complications.
Support management in infective-endocarditis surgery
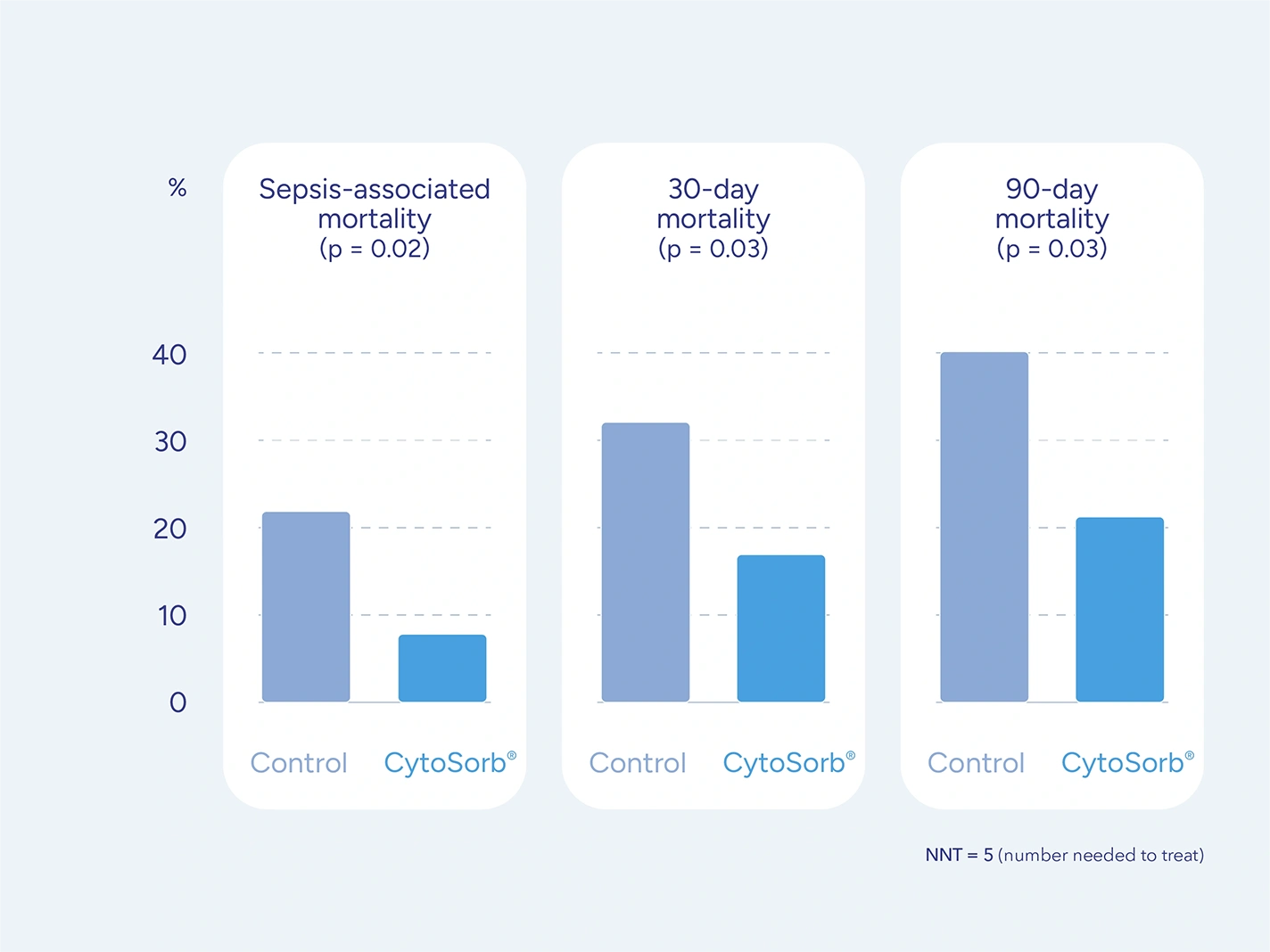
Although infective-endocarditis surgery is a curative intervention, it can be associated with bacterial dissemination and immune activation, potentially contributing to hemodynamic instability and multi-organ dysfunction.
Clinical reports have described reduced cytokine levels, modulation of inflammatory mediators, and improved hemodynamic parameters in selected cases.

Infective Endocarditis
General Treatment Goals
CytoSorb is intended to lower excessive levels of cytokines in the patients blood. This therapeutic measure can support management of local and systemic inflammatory processes in patients with infective endocarditis.
Support aortic surgery patients
Complex aortic surgery can be associated with dysregulated immune responses and complications such as vasoplegic shock or multi-organ dysfunction.
CytoSorb Therapy is designed to reduce elevated levels of cytokines, aiming to support hemodynamic stability and assist in managing hyperinflammatory conditions during and after surgery.

Aortic surgery
General Treatment Goals
CytoSorb is intended to lower excessive cytokine levels and blood thinning agents such as Ticagrelor or Rivaroxaban in patients undergoing on-pump cardiac surgery. These therapeutic measures can support reduction of bleeding risks and management of local as well as systemic inflammatory processes.
Support stabilization in heart failure patients
Heart failure patients are at increased risk for cardiogenic shock and vasoplegia.
CytoSorb® Therapy is designed to adsorb elevated cytokine levels, supporting hemodynamic stabilization in critically ill patients as part of a multimodal approach.

Managing postoperative hyperinflammation
In postoperative settings, CytoSorb® Therapy to remove cytokines is designed to support the management of systemic hyperinflammation and help stabilize hemodynamics.
Support hemodynamics
An uncontrolled inflammatory response after surgery plays a significant role in post-op morbidity or mortality, contributing to vasoplegic shock and multi-organ dysfunction.
CytoSorb is indicated for the removal of cytokines which may in turn contribute to the management of hyperinflammation and to hemodynamic stabilization.

Best practice therapy management
Supporting ECMO patient management
Patients on ECMO frequently exhibit signs and symptoms of hyperinflammation. CytoSorb® intended to remove elevated cytokines may help modulate the systemic inflammatory response.
ECMO with CytoSorb
ECMO is increasingly used in ARDS and cardiogenic shock, but morbidity and mortality rates remain high.
Treatment options to enhance the clinical benefits of ECMO support and prevent complications, such as ongoing hyperinflammation, are currently limited.
Hyperinflammation is frequently present during ECMO support and CytoSorb with its approved use for removal of elevated cytokines may contribute to the overall efforts to control the systemic inflammatory response.

Supporting VA-ECMO management
VA-ECMO supports macro-circulatory hemodynamics, but may introduce circulatory stress. Published clinical experience has described associations with accelerated stabilization of hemodynamics when CytoSorb® is used as part of ECMO management.
Supporting VV-ECMO management
- Akil et al., Thorac Cardiovasc Surg 2021; 69(3):246-251
- Akil et al., J Clin Med 2022; 11(20):5990
- Angheloiu et al., JACC Basic Transl Sci 2017; 2(2):135-145
- Bernardi et al., Crit Care 2016; 20(1):96
- Boss et al., PLoS One 2021; 16(2):e0246299
- Bottari et al., Int J Art Organs 2020; 43(9):587-593
- Calabrò et al., Artif Organs 2019; 43(2):189-194
- Cao et al., Eur Heart J 2023; 44(20):1780-1794
- Cohen et al., Am J Cardiovasc Drugs 2023; 23(4):429-440
- David et al., J Intensive Care 2017; 5(1):12
- Diab et al., 2022; 145(13):959-968
- Doukas et al., J Clin Med 2023; 12(2):546
- Gleason et al., Sem Thorac Cardiovasc Surg 2019; 31(4):783-793
- Gruda et al., PLoS One 2018; 13(1):e0191676
- Guiterrez et al., Cir Cardiov 2024; 31:56-63
- Jansen et al., Crit Care 2023; 27:117
- Javanbakht et al., PharmacoEconomics Open 2020; 4:307-319
- Haidari et al., Ann Thorac Surg 2020; 110(3):890-896
- Haidari et al., PLoS One 2022;17(7):e0266820
- Haidari et al., ICV&TS 2023; 36(1):ivad010
- Haidari et al., Ann Thorac Surg 2023; 11:3068
- Hassan et al., JTCVS Open 2023;15:190-196
- Hassan et al., Annals of Thoracic Surg 2019; 108(1):45-51
- Hassan et al., Ann Thorac Cardiovasc Surg 2022;28(3):186-192
- Hayanga et al., Int Care Med Exp 2022; 10(40):494
- Holmen et al., J Cardiothorac & Vasc Anesth 2022; 36(8 Pt B):3015-3020
- Koertge et al., Blood Purif 2018; 45(1-3):126-128
- Kalisnik et al., J Clin Med. 2022;11(14):3954
- Kuehne et al., Int J Artif Organs 2019;42(4):194-200
- Mehta et al., Interdiscip Cardiovasc Thorac Surg 2024; 38(4):ivae050
- Mehta et al., Cardiothorac Vasc Anesth 2021; 35(2):673-675
- Naruka et al., Heart Lung Circ 2022; 31(11):1493-1503
- Nemeth et al., J Clin Trans 2018; 32(4):e13211
- Nemeth et al., ESC Heart Fail 2024;11(2):772-782
- NICE Medtech innovation briefing, February 2021; www.nice.org.uk/guidance/mib249
- Piskovatska et al., Healthcare 2023; 11(3):310
- Poli et al., Crit Care 2019; 23:108
- Rao et al., J Cardiovasc Dev Dis 2023; 10(9):366
- Røed-Undlien et al., Int J Surg 2024; 110(12):7782-7790
- Rugg et al., Biomedicines 2020; 8(12):539
- Saller et al., Eur J Cardiothorac Surg 2019; 56(4):731-737
- Schmoeckel et al., J Thromb Thrombolysis 2024; epub (doi; 10.1007/s11239-024-02996-x)
- Singh et al., Am J Case Rep 2023; 24:e940383
- Soltesz et al., J Clin Med 2022; 11(21):6517
- Thielmann et al., Indian J Thorac Cardiovasc Surg 2024; 40(Suppl 1):69-77
- Traeger et al., Int J Artif Organs 2020; 43(6):422-429
- Traeger et al., Int J Artif Organs 2017; 40(5):240-249
CytoSorb 300 IFU 03/2023 – Indications:
CytoSorb is indicated for use in conditions where elevated levels of cytokines and/or bilirubin and/or myoglobin exist. CytoSorb is indicated for use intraoperatively during cardio-pulmonary bypass surgery for the removal of P2Y12-Inhibitor Ticagrelor and/or Factor Xa-Inhibitor Rivaroxaban. Results from current studies suggest that CytoSorb may be administered for up to 7 consecutive days. Maximum Treatment Time per Device: 24 Hour.
NON US HEALTHCARE PROFESSIONALS AREA
This section of the website contains medical and clinical information regarding products that are not cleared or approved by the U.S. Food and Drug Administration (FDA).
Under U.S. federal law, including the Federal Food, Drug, and Cosmetic Act (FD&C Act), dissemination of information about medical devices that are not FDA-cleared or approved for use in the United States may constitute unlawful promotion or misbranding if directed to U.S. healthcare professionals.
Accordingly:
This content is intended solely for Healthcare Professionals located outside the United States and Canada.
The products described are not approved or cleared in the United States or Canada for any indication.
Access by U.S. Healthcare Professionals is not permitted.
BY PROCEEDING, YOU CONFIRM THAT YOU ARE:
A licensed Healthcare Professional, and located outside the United States and Canada.
Physician experience, clinical practice patterns, risks, and patient outcomes may vary. The information presented reflects individual clinical experience and does not constitute medical advice.